Abstract:
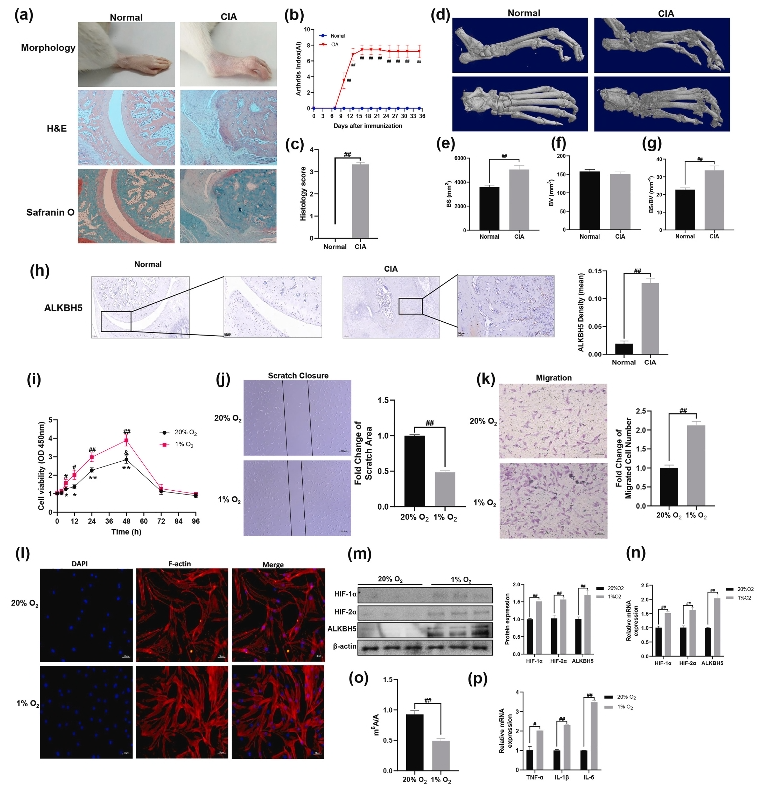
Previous studies have shown that epigenetic factors are involved in the occurrence and development of rheumatoid arthritis (RA). However, the role of N6-methyladenosine (m6A) methylation in RA has not been determined. The aim of this study was to investigate the role and regulatory mechanisms of hypoxia-induced expression of the m6A demethylase alkB homolog 5 (ALKBH5) in RA fibroblast-like synoviocytes (FLSs). Synovial tissues were collected from RA and osteoarthritis (OA) patients, and RA FLSs were obtained. ALKBH5 expression in RA FLSs and collagen-induced arthritis (CIA) model rats was determined using quantitative reverse transcription-PCR (qRT–PCR), western blotting and immunohistochemistry (IHC). Using ALKBH5 overexpression and knockdown, we determined the role of ALKBH5 in RA FLS aggression and inflammation. The role of ALKBH5 in RA FLS regulation was explored using m6A-methylated RNA sequencing and methylated RNA immunoprecipitation coupled with quantitative real-time PCR. The expression of ALKBH5 was increased in RA synovial tissues, CIA model rats and RA FLSs, and a hypoxic environment increased the expression of ALKBH5 in FLSs. Increased expression of ALKBH5 promoted the proliferation and migration of RA-FLSs and inflammation. Conversely, decreased ALKBH5 expression inhibited the migration of RA-FLSs and inflammation. Mechanistically, hypoxia-induced ALKBH5 expression promoted FLS aggression and inflammation by regulating CH25H mRNA stability. Our study elucidated the functional roles of ALKBH5 and mRNA m6A methylation in RA and revealed that the HIF1α/2α-ALKBH5-CH25H pathway may be key for FLS aggression and inflammation. This study provides a novel approach for the treatment of RA by targeting the HIF1α/2α-ALKBH5-CH25H pathway.
Key Words:
ALKBH5;;CH25H;;Hypoxia;;m6A;;Rheumatoid arthritis