On April 7th, a joint effort between the Biotechnology Research Institute's Microbial Protein Design and Intelligent Manufacture team and the Institute of Animal Science's feed enzyme engineering team developed a strategy to predict the optimal temperature of proteins using a combination of artificial intelligence and molecular evolution. This strategy was used to design and screen out the positive mutants of the PET hydrolase, LCCICCG, which have the capability to break down highly crystalline PET plastics. This research was reported in the Journal of Hazardous Materials.
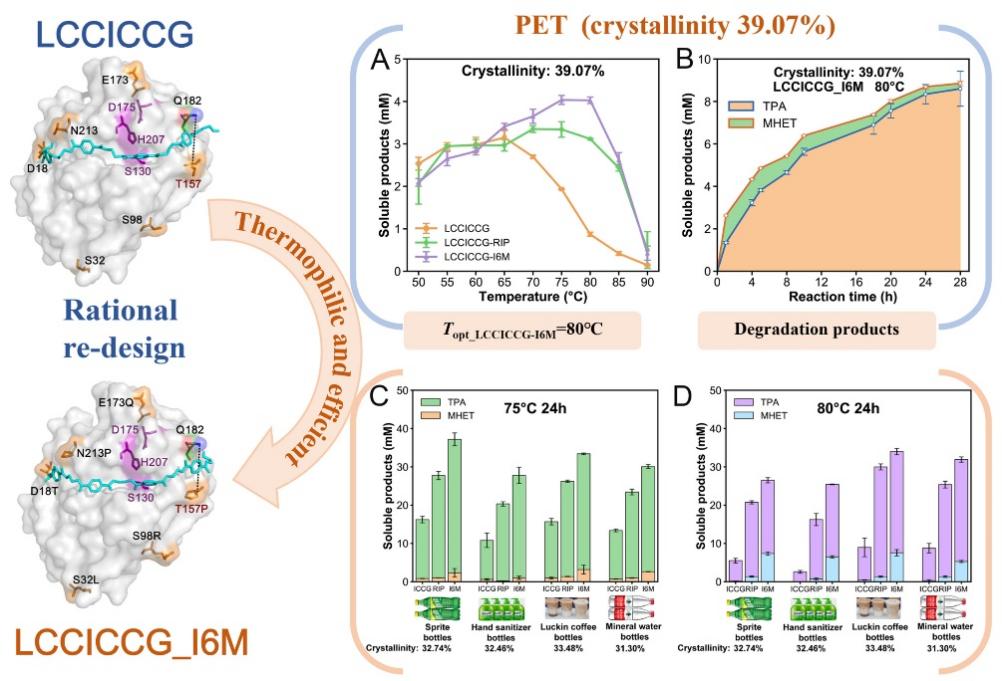
Plastics are all around us and cause serious environmental damage. Polyethylene terephthalate (PET) is one of the most common plastics that can be broken down by PET hydrolase. However, many of the existing PET hydrolases can only degrade amorphous or pre-treated PET plastics with low crystallinity, and they require relatively low temperatures. The most thermophilic PET hydrolase, LCCICCG, has been found to be able to degrade 90% of PET plastics (crystallinity: 14.6%), but it is not as effective in breaking down highly crystalline PET plastics, which are commonly used with a crystallinity of 30%-50%. Therefore, it is essential to develop new thermostable enzymes that can effectively degrade high-crystallinity PET plastics.
In this study, researchers used the LCCICCG as the subject and employed a deep learning-based prediction tool, Preoptem, along with different evolutionary analytical methods. After virtual screening of mutants and experimental validation, a six-point mutant, LCCICCG-I6M, was finally obtained. The optimum temperature of LCCICCG-I6M for degradation PET powder (crystallinity:39.07%) was increased from 65°C (LCCICCG) to 75-80°C. In validation experiments on commercial PET products, the degradation efficiency was 49.08 ± 0.16% at 75 °C and 10 h towards commercial PET disposable trays (crystallinity:14.8%) under laboratory conditions. The degradation efficiency for four more commonly used commercial bottle-grade PET plastics with high crystallinity (Sprite brand soft drink bottles, Blue Moon hand soap bottles, RuiXing brand coffee cups and mineral water bottles with crystallinity >30%) was significantly higher than that of wild type at 75°C and 24 h and 80°C and 24 h, respectively. This study provides a new method and material for green degradation of high crystallinity PET plastics.
Zundan Ding, a PhD student at the Biotechnology Research Institute, and Guoshun Xu, a PhD student at the Institute of Animal Science, are co-first authors of this paper; Jian Tian and Feifei Guan, at the Biotechnology Research Institute, and Huoqing Huang, at the Institute of Animal Science, are co-corresponding authors. This work was supported by the National Key R&D Program of China and the Central Public-interest Scientific Institution Basal Research Fund.
|