Recently, a research group led by Yuquan Xu, from the Biotechnology Research Institute, CAAS published an article on Journal of the American Chemical Society (a top-ranking journal in the field of chemistry, IF 14.357) on February 15, 2019. This study describes the rational design and reprogramming of the regioselectivity of two fungal OMTs (LtOMT and HsOMT), and the application of engineered OMTs in the combinatorial biosynthetic tailoring of drug-like small molecules.
O-Methylation modulates the pharmacokinetic and pharmacodynamic (PK/PD) properties of drug-like natural products affecting their bioavailability, stability, and binding to targets. Diversity-oriented combinatorial biosynthesis of new chemical entities for drug discovery, and optimization of known bioactive scaffolds during drug development both demand efficient O-methyltransferase (OMT) biocatalysts with considerable substrate promiscuity and tunable regioselectivity that can be deployed in a scalable and sustainable manner.
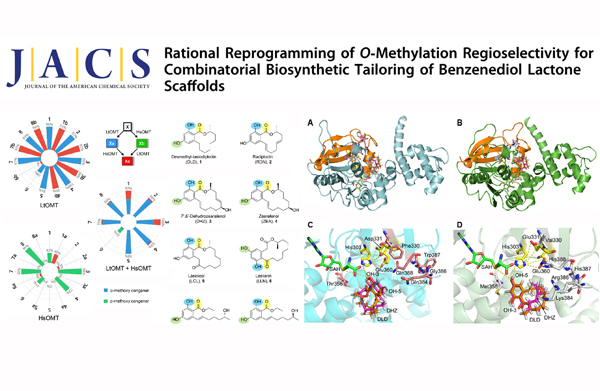
Figure. Combinatorial tailoring with LtOMT and HsOMT (left). Structure modeling and molecular docking of LtOMT and HsOMT (right).
O-methylation is used by nature and medicinal chemistry for the hydrophobic masking of peripheral hydroxyl or carboxylic acid moieties to adjust the physicochemical and biological properties of bioactive compounds. As opposed to chemical synthesis, enzymatic O-methylation offers regio- and stereospecific outcomes even for highly complex and reactive scaffolds without resorting to expensive multistep protection/deprotection strategies. Extensive studies have been conducted on the identification and engineering of bacterial and plant OMTs during the past decades. However, little attention has been paid up to now to define the substrate promiscuity and modulate the regioselectivity of fungal secondary metabolite OMTs, despite these enzymes constituting the most common tailoring activities during the biosynthesis of such natural products. The authors of this article employed an efficient total biosynthetic and biocatalytic platform featuring a pair of fungal OMTs with orthogonal regiospecificity to produce unnatural O-methylated benzenediol lactone (BDL) polyketides, and reprogramed the regioselectivity of these enzymes by structure-guided active site cavity engineering. Their findings will guide combinatorial biosynthetic tailoring of unnatural products towards the generation of diverse chemical matter for drug discovery and the PK/PD optimization of bioactive scaffolds for drug development.
More details of this study are available on the links below:
https://pubs.acs.org.ccindex.cn/doi/10.1021/jacs.8b12967.
|