Recently, the Protein Design and Intelligent Manufacturing team from the Biotechnology Research Institute (BRI) of the Chinese Academy of Agricultural Sciences (CAAS), in collaboration with the Institute of Animal Science (IAS) of CAAS and Wenzhou Medical University, has developed a novel vaccine platform based on mesoporous silica nanoparticles (DMONs) framework. This platform enables the co-loading and delivery of antigen and adjuvant, resulting in a vaccine with higher immunogenicity and a longer duration of protection. The related research has been published in Materials Today Bio.
Infectious diseases have always posed a persistent threat to humans and animals, with recent examples like COVID-19 and African Swine Fever serving as stark reminders. Since the advent of cowpox vaccination in the 18th century, vaccines have protected and saved hundreds of millions of lives and immense economic value, becoming one of the most cost-effective and efficient means for preventing and controlling infectious diseases. Adjuvants, discovered in the 1920s and derived from the Latin adjuvare (meaning to help or aid), are indispensable components of vaccine formulation. They are broadly categorized into potentiators (activating the immune system) and delivery systems (promoting antigen retention and presentation). Since the beginning of this century, adjuvant development has surged, with related research advancing rapidly.
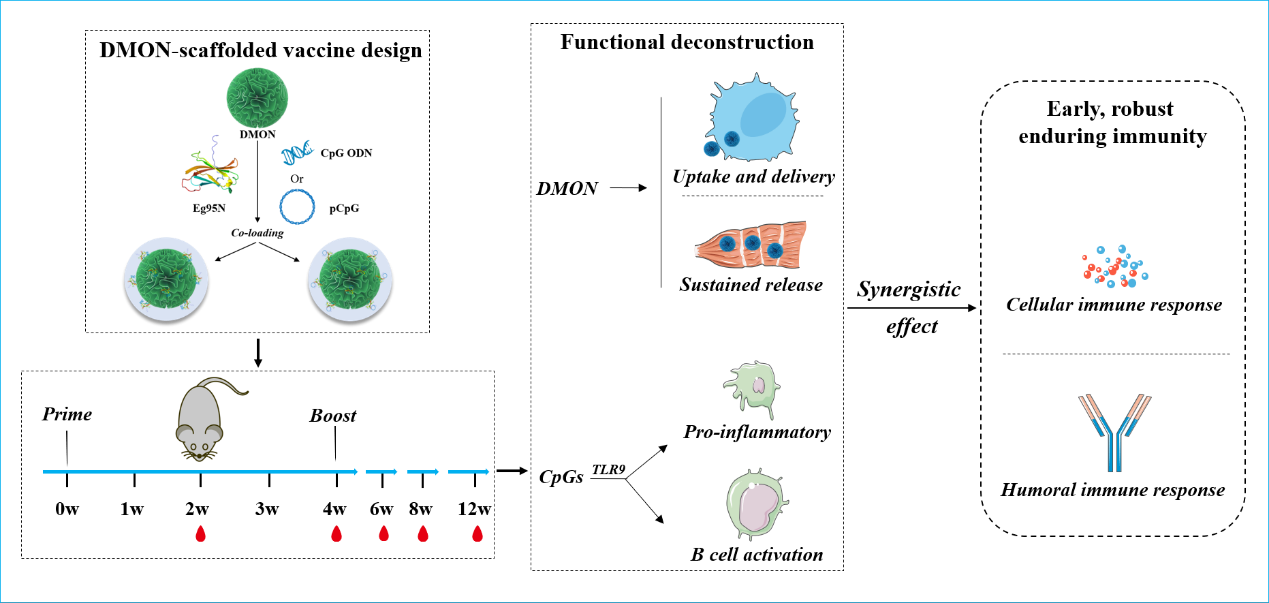
Building on their previous work with ferritin nanoparticles (Int J Nanomedicine, 2025), the research team optimized the synthesis of highly loadable and safe DMONs featuring central-radial fibrous channels. Employing an "All-in-one" design strategy, they successfully co-loaded an antigen (protein) and an adjuvant (nucleic acid) onto DMONs to construct a novel nanovaccine. This vaccine not only facilitates cellular uptake, sustained release, and delivery of both the antigen and adjuvant but also enhances the activation of the immune system, thereby improving antigen utilization efficiency. This dual approach ultimately enables the vaccine to induce earlier, stronger, and longer-lasting immunity, providing robust technical support for the development of both veterinary and human vaccines.
Professor Yinü Li from BRI, CAAS, and professor Jiaxi Ru from Wenzhou Medical University are the co-corresponding authors of the paper. Associate professor Ting Xin from IAS, CAAS, and associate professor Xintao Gao from BRI, CAAS are the co-first authors.
The full text of the paper is available at: https://doi.org/10.1016/j.mtbio.2025.101868
|