Abstract:
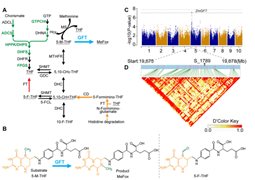
Identifying genes involved in folate accumulation is critical for elucidating the regulatory mechanisms of folate metabolism and breeding folate-rich crops. Here, a natural A-to-G variation at the 682nd bp is identified in the coding sequence of an identified plant gene glutamate formiminotransferase (GFT) in maize, leading to a glycine-to-asparagine substitution at the 228th in the protein sequence and contributing to the variation of folate accumulation in mature seeds of a maize inbred line population. This gene encodes a protein highly similar to the formiminotransferase domain of mammalian formiminotransferase cyclodeaminase. In vitro biochemical analysis of this protein reveals an activity of triggering 5-methyl-tetrahydrofolate (5-M-THF)-to-MeFox conversion, other than exerting an activity of formiminotransferase in mammals. Loss of ZmGFT function triples 5-M-THF levels, and overexpression of G-allele-carrying ZmGFT boosts the metabolic flow toward MeFox. Functional conservation of GFT is validated in rice and Arabidopsis. The asparagine-to-glycine substitution enhances 5-M-THF-to-MeFox conversion, as demonstrated by in vitro assays and in silico analyses. The functional characterization of the GFT gene has uncovered a new metabolic fate of 5-M-THF, apart from a C1 donor for methionine synthesis, in plants, and a distinct activity from its mammalian ortholog. The natural variation identified is useful for breeding folate-fortified maize varieties.
|