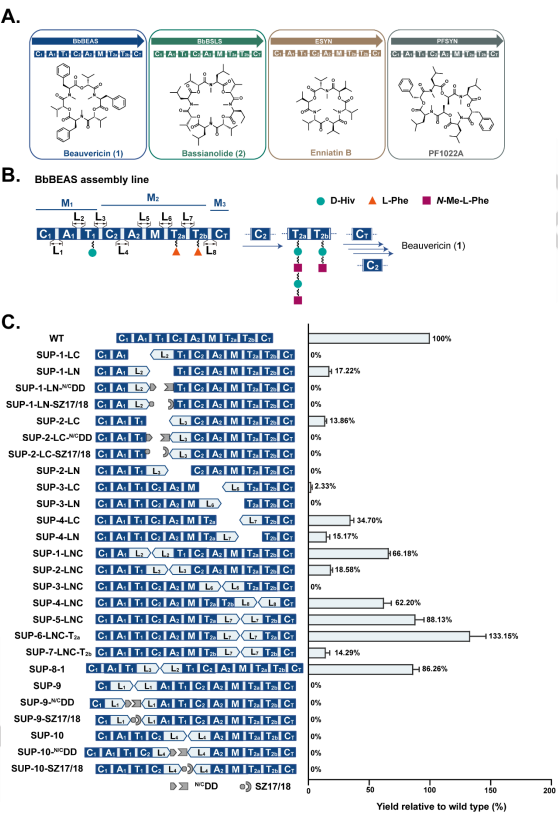
Abstract:
Unnatural product (uNP) nonribosomal peptides promise to be a valuable source of pharmacophores for drug discovery. However, the extremely large size and complexity of the nonribosomal peptide synthetase (NRPS) enzymes pose formidable challenges to the production of such uNPs by combinatorial biosynthesis and synthetic biology. Here we report a new NRPS dissection strategy that facilitates the engineering and heterologous production of these NRPSs. This strategy divides NRPSs into “splitting units”, each forming an enzyme subunit that contains catalytically independent modules. Functional collaboration between the subunits is then facilitated by artificially duplicating, at the N-terminus of the downstream subunit, the linker - thiolation domain - linker fragment that is resident at the C-terminus of the upstream subunit. Using the suggested split site that follows a conserved motif in the linker connecting the adenylation and the thiolation domains allows cognate or chimeric splitting unit pairs to achieve productivities that match, and in many cases surpass those of hybrid chimeric enzymes, and even those of intact NRPSs, upon production in a heterologous chassis. Our strategy provides facile options for the rational engineering of fungal NRPSs and for the combinatorial reprogramming of nonribosomal peptide production
|